Limiting Carbon Whisker Formation for SMR

Steam methane reforming (SMR, eqn 1) is one of the most industrially adopted reaction for designing a hydrogen rich synthesis gas (CO + H2) generation process. Depending on the desired end application of the synthesis gas and the required H2 concentration it may be accompanied by water gas shift (WGS, eqn 2) reaction which enriches the product stream with H2. SMR is highly endothermic in nature and the heat required for this reaction is usually provided by methane combustor which helps generate superheated steam. The small amount of heat generated from the WGS reaction can also be used if the reactor system is designed to capture that heat.
CH4+ H2O → 3H2+ CO (∆H = +206 kJ/mol) [1]
H2O + CO → CO2+ H2 (∆H = -41.16 kJ/mol) [2]
The operating conditions of the process vary from process to process but usually the system is operated at 20-30bar pressure with the exit gas temperature of about 850°C. In the reaction bed near the reactor wall the catalyst instantaneous temperature can be as high as 950°C.
The high H2containing gas stream can be used as an additive in petrochemical refineries that use H2to upgrade the crude oil to manufacture high value fuels such as gasoline, diesel, and jet fuel. It can be used as a raw material in ammonia and methanol manufacturing plans. High purity hydrogen can also be used as fuel for conversion to electrical energy in fuel cell systems.
The process system for SMR and hydrogen generation has been in operation for decades. However, it still faces some technical challenges that need to be addressed. One such challenge is to resist catalytic deactivation as a result of certain demanding reaction conditions. Deactivation of a catalyst is characterized by a time dependent decay in the catalysts’ performance as a result of poison adsorption, strengthening of the metal-support interaction, coking, and sintering. Poison adsorption is usually limited by removal of S from the natural gas feed before the reforming step. High metal support interaction, which can be beneficial in applications like automotive TWC where O from Ce helps in combustion of HC at the metal site under rich conditions [link to our CZO and TWC press release], may turn detrimental to the SMR Ni catalyst performance by formation of NiAl2O4which significantly inhibits Ni ability to activate CH4and thus its SMR activity. This can be countered by addition to basic promoters that limit the NiAl2O4formation.
Among the various deactivation mechanisms, coking is one of the most prominent mechanism causing catalytic performance degradation. The carbon formed on the catalyst could be filamentous, encapsulating, or graphitic in nature. Filamentous carbon grows on active catalytic metal like Ni, fouling the metal, then propagates through the catalyst pores, thus blocking it, increasing mechanical stress on the catalyst pellet resulting in disintegration of the catalytic pellets. Filamentous carbon may not always accompany immediate catalytic activity loss, but the disintegration of the pellets produces finer pellet particles which create back pressure in the reactor vessel rendering the SMR process inoperable. Step edge sites on the Ni crystallites are believed to be acting as nucleation sites for C whiskers and the catalyst can resist carbon whiskers if it maintains a smaller crystal size by maximizing Ni dispersion.
Pyrochem Catalyst Company (PCC) has a proprietary pyrochlore catalyst technology (PyroCat™) that can incorporate catalytically active metals in the pyrochlore crystal lattice. This isomorphic substitution of the metal allows it to be active while maintaining very high dispersion, and resistance to sintering at elevated temperatures. PCC has developed highly stable Rh substituted PyroCat™ which have been tested for diesel reforming reaction under high S environments at 900°C for fuel cell applications. This experience in designing of sintering resistant catalyst has allowed PCC to develop a Ni PyroCat™ with extremely high resistance to C whisker formation.
To test for the efficacy of the Ni PyroCat™ towards limiting C whisker, we performed SMR test under conditions that would promote C formation (S:C = 0.9, T = 850°C, P = 15bar). Following the SMR test, a temperature programmed reduction (TPR) was conducted to hydrogenate the surface C. Different allotropes of C have signature temperatures at which they hydrogenate with C whisker showing a signature reduction peak at ~620°C. To obtain a fair comparison to Ni PyroCat, a commercially available SMR catalyst was also tested under identical conditions. The comparative graph with the TPR profiles for commercial SMR catalyst and Ni PyroCat in presented in Fig. 1.

Fig. 1. Comparative graph showing the TPR profile of spent commercial SMR catalyst and spent Ni PyroCat after SMR (S:C = 0.9, T = 850°C, P = 15bar).
The TPR profiles (Fig. 1) clearly indicates the unique ability of the Ni PyroCat to kinetically resist C whisker formation during SMR under C inducing conditions compared to what a commercially available catalyst is capable of. To truly demonstrate the quality of the Ni PyroCat, PCC wanted to challenge the catalyst further by promoting Ni sintering prior to running SMR under the above-mentioned conditions. To facilitate metal sintering, the Ni PyroCat to was by high pressure and high temperature steam reduction. In this aging test, the catalyst was reduced in 4% H2, 30% H2O, and 66% Ar at 850°C and 5 bar for 12 hrs. This is similar to the aging test run industrially for screening SMR catalysts’ ability to withstand extended duration of operation. The catalyst after the hydrothermal reduction will be addressed as “Aged Ni PyroCat”. This aged Ni PyroCat was then tested for SMR under similar conditions as above. The TPR and of the aged catalyst after SMR is shown in Fig. 2.
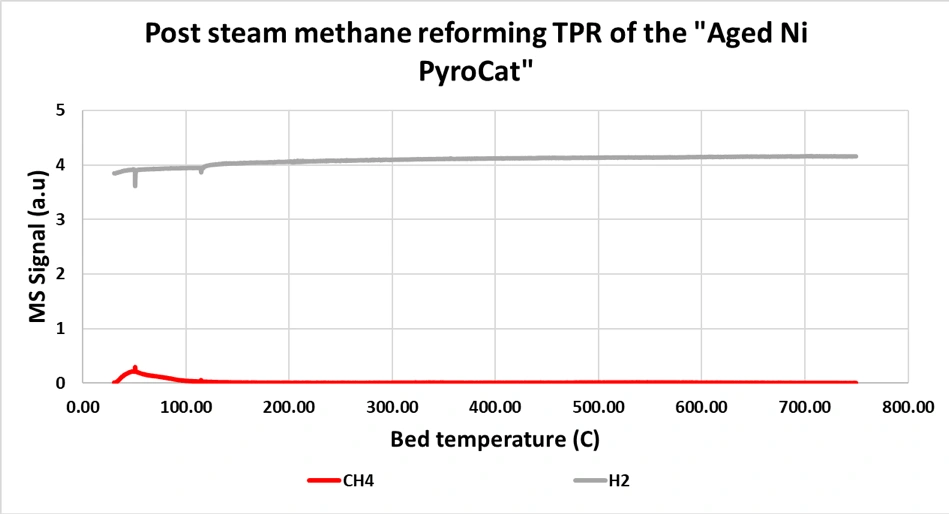
Fig. 2. The steam aged catalyst was tested for SMR and the C formed during SMR was characterized by TPR. This graph is that TPR profile.
Fig. 2 does not show any sign of CH4being formed during the entire temperature range. The commercial catalyst was not hydrothermally aged since it was unable to limit carbon under fresh conditions, it is unlikely it would have performed better after sintering. The lack of methanation during the TPR of the aged Ni PyroCat suggests that either (a) there is no C formed over the catalyst during SMR, or (b) if there is some minimal C formed, it is not in the vicinity of any methanation site or Ni cluster. Considering Ni clusters are nucleation sites for C whisker, either of the above hypothesis suggests that the Ni PyroCat can incorporate Ni in the pyrochlore crystal lattice in a way that it precludes the formation of Ni cluster and thus prohibits the formation of C whiskers.
To advance this work on developing a C whisker resistant Ni PyroCat, PCC is working on subjecting the catalyst to even harsher aging conditions and test its C whisker limiting ability in collaboration with our industrial partners. The next step is to age the catalyst further and optimize the structural stability to enhance the robustness of the Ni PyroCat to make it applicable to a wide range of hydrogen or syngas generation processes.
To know more about how Ni PyroCat could help meet your hydrogen or syngas generation needs, feel free to contact us at jbharrison@pyrochemcatalyst.com.

